Our Phase II & III Capabilities
Many of our sites have participated in research for 10+ years. Our partnerships provide access to CA residents throughout the State with nearly 26 partnerships in over 50+ locations. We help provide access to a diverse patient population, including minority groups that otherwise go unrepresented in research.

Rapid Start
- Central IRB.
- Contract and budget response within 48 hours.
- Regulatory documents completed in parallel.
- Often able to complete start-up within 2 weeks.
- Dedicated Call Center team to assist with enrolling.

Capabilities
- FibroScans, ultrasounds, X-rays, PBMC, local labs, and much more available at many locations.
- Nearby access to liver biopsy, MRI-PDFF, DEXA, CT-Scans, and much more.
- Efficient prescreening process.
- Experience with telemedicine, virtual, and hybrid trials.

Quality Control
- Our Internal quality team provides QC on all ICF and critical documents.
- eRegulatory and eSource offer increased quality and efficiency.
- eDocuments available for sponsor review 24/7.
Allergy & Immunology
Cardiology
Dermatology
Family Medicine
Gastroenterology
Infectious Disease
Internal Medicine & Vaccines
Nephrology
Neurology
Obstetrics & Gynecology
Oncology
Pediatrics
Ophthalmology
Retina
Rheumatology
Urology
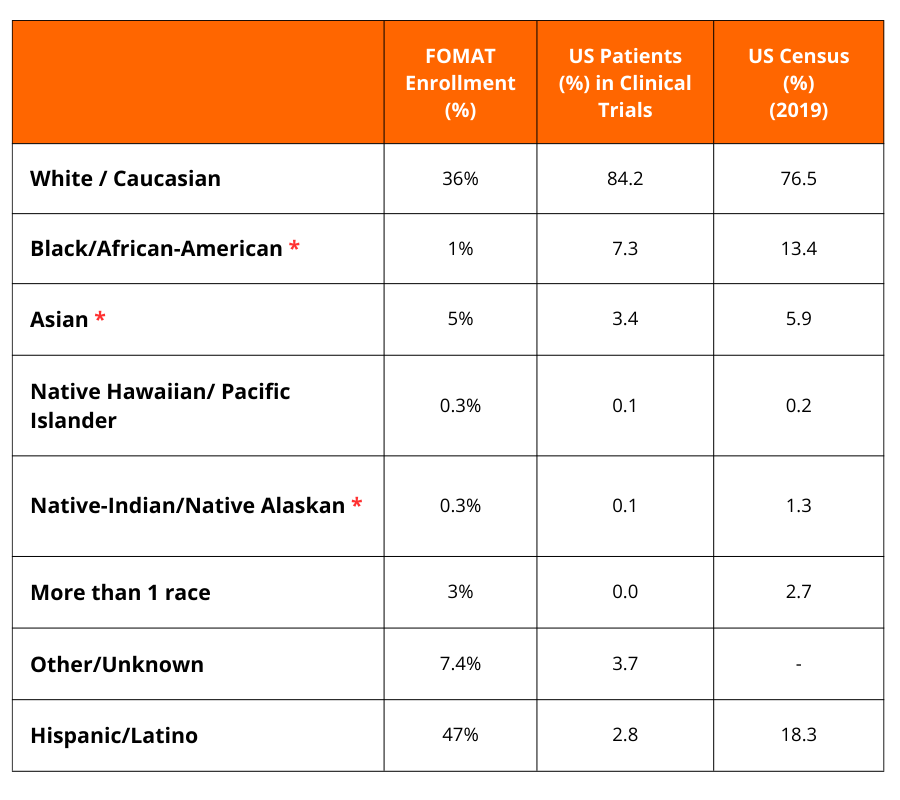
Our Diversity Data