Diversity in Clinical Trials
Innovating Healthcare
For All
Why Diversity In Clinical Trials Matters
Diversity in clinical trials is crucial. Including people of different races, ethnicities, ages, and genders helps ensure treatments are safe and effective for everyone. This variety helps researchers understand how different people might react to medicines or vaccines. It's about making sure health solutions work well for all communities, leading to better, safer healthcare for everyone.
The Importance of Diverse Research in Clinical Trials
Diversity in clinical trials is crucial for understanding how different factors like age, gender, weight, race, and ethnicity influence medication responses. The FDA states, "Diversity...is critical to understanding the therapy’s benefit-risk profile across all users." This ensures treatments are safe and effective for all, leading to healthcare that benefits everyone.
Our Commitment to Diversity
Since 2013, FOMAT Medical Research has prioritized diversity by choosing diverse trial sites and reaching out to underrepresented communities. Our efforts include providing accessible, culturally sensitive materials to ensure our clinical research is inclusive. This commitment has significantly improved the enrollment and representation of these groups in trials, reflecting our dedication to innovating healthcare through diversity and making our research truly representative of all communities.
* Our dedication was recognized with our top 3 finalist position for the 2022 SPRIA Award.
FOMAT's Approach to Enhancing Diversity in Clinical Trials
FOMAT is dedicated to tackling the significant challenges that impede diversity in clinical research, such as underrepresentation, insufficient awareness or access, cultural and linguistic barriers, and community distrust. To surmount these hurdles, FOMAT employs strategic recruitment efforts, fosters community involvement, and creates materials that are sensitive to various cultural contexts. These initiatives underscore FOMAT's unwavering dedication to removing obstacles that hinder participation.
Access to Diverse Population
Patient Recruitment Strategy
Site Management Activities
Track Progress
Our Achievements in Diversity
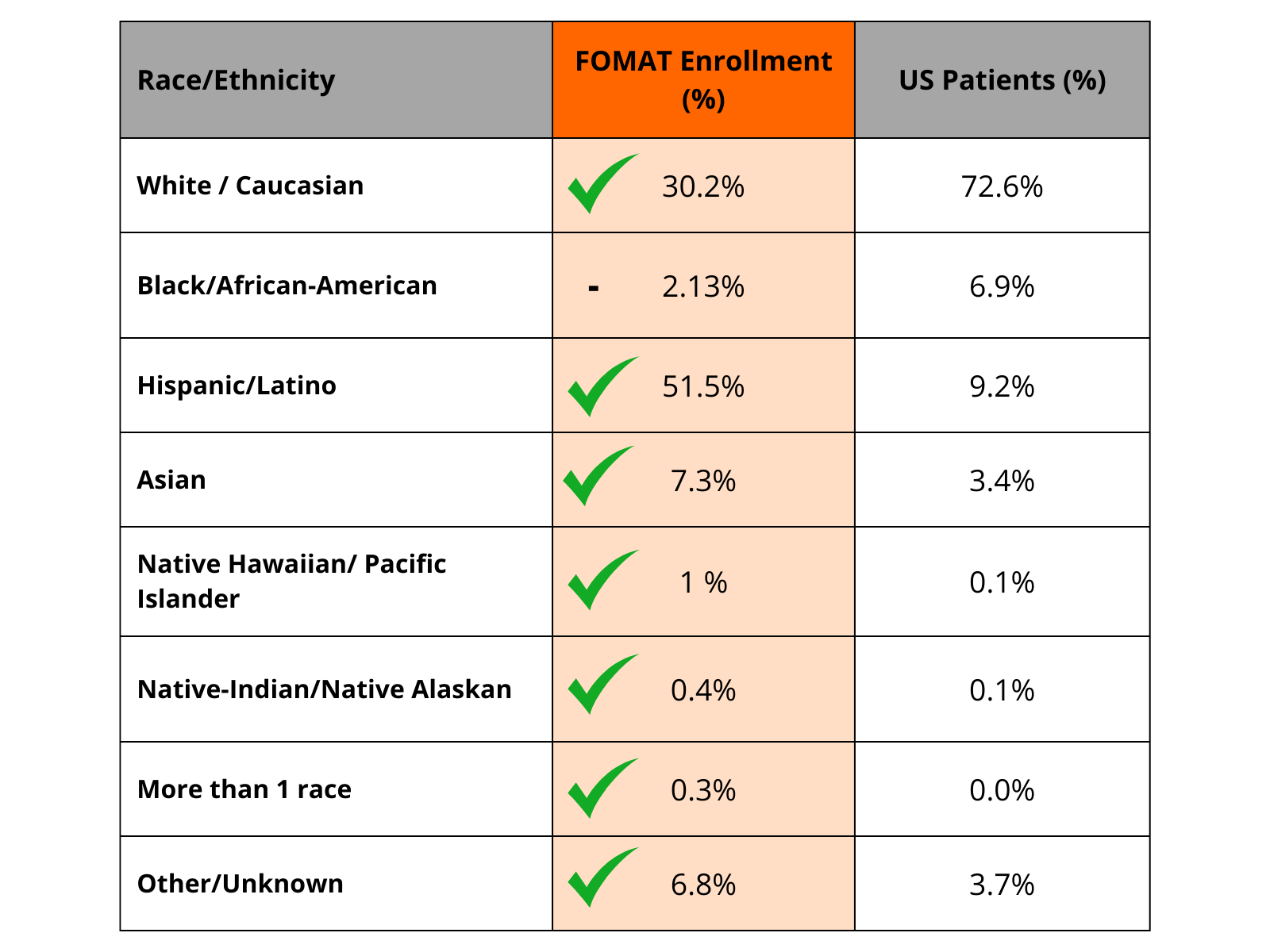
* We are actively working to further engage and bring clinical trials to this underrepresented minority group.
Phase 1 Unit
Navigate early-stage trials with us, focusing on safety, dosage determination, aiding the path of your novel drug development.
Our Phase II & III capabilities
We have partnered with the largest groups in California to run successful embedded clinical research studies.
Our Vaccine
Capabilities
With over 20 years of experience in clinical trials and having access to a highly diverse patient population-we are perfectly positioned to assist organizations throughout the vaccine development process.
