Pediatric Patients
Showing all 6 results
-
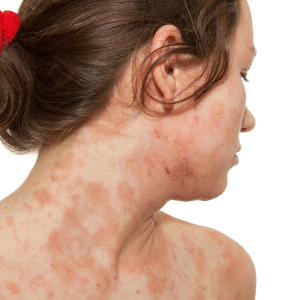
Atopic Dermatitis
This clinical trial aims to explore and evaluate biomarkers associated with Atopic Dermatitis in individuals diagnosed with the condition. Criteria & Qualifications: • Open to males and females in good general health. • Individuals willing to participate and follow the study protocol. • Participants who can provide relevant medical records. Compensation: Contact us for more information
Atopic Dermatitis
Read more -
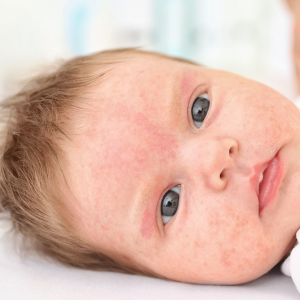
Atopic Dermatitis Study
This clinical trial is being conducted to evaluate biomarkers of Atopic Dermatitis in pediatric patients whose disease is not adequately controlled with topical prescription therapies or when those therapies are not medically advisable. Criteria & Qualifications: • Males and Females in good general health. • Patients with moderate to severe AD whose disease is not adequately controlled with topical therapies. • Must be 0 years to 11 years old. Compensation: Contact us for more information
Atopic Dermatitis Study
Read more -

Pediatric Respiratory Syncytial Virus (RSV)
We’re conducting a study to explore a new option for children with respiratory syncytial virus (RSV). This research aims to better understand how it can support recovery and overall health. Criteria & Qualifications: • Must be under 18 years old. • Diagnosed with RSV. • May have health factors like certain medical conditions. Compensation: Contact us for more information
Pediatric Respiratory Syncytial Virus (RSV)
Read more -
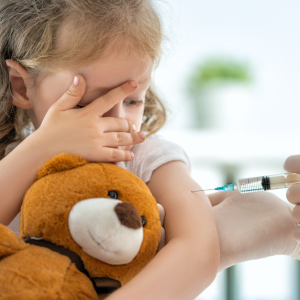
Pneumococcal Vaccine
We’re conducting a study to evaluate vaccines for infants to enhance their protection against common illnesses. Your baby's participation could contribute to advancing pediatric care while receiving routine vaccinations. Criteria & Qualifications: • Infants aged 2 to 3 months. • Healthy and medically stable. • Born full-term or preterm but meeting weight requirements. Compensation: Contact us for more information
Pneumococcal Vaccine
Read more -

Pneumococcal Vaccine Study
Support groundbreaking research by participating in our Pneumococcal Vaccine Study. Your involvement could help improve vaccine protection for children everywhere. Criteria & Qualifications: • Your child is eligible if they meet the study's age requirements. • Able to commit to 7–8 study visits over a period of 16–19 months. • Parent or guardian must be able to attend all 7–8 study visits over 16–19 months. Compensation: Contact us for more information.
Pneumococcal Vaccine Study
Read more