Imagine your body as a bustling city where everything works seamlessly, maintaining the delicate balance of health. Now, think of non-alcoholic steatohepatitis (NASH) or Metabolic dysfunction-associated steatohepatitis (MASH) as an insidious construction project gone wrong inside this city, gradually laying down unwanted infrastructure—fibrosis—within the liver. Over time, without intervention, this can escalate to severe stages of liver fibrosis and even cirrhosis, silently escalating to life-threatening complications. Nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) orMetabolic dysfunction-associated steatohepatitis (MASH) are common conditions with a rising burden1.
Experts estimate about 24% of U.S. adults have NAFLD and about 1.5% to 6.5% of U.S. adults have NASH/MASH 2.
Millions of people are currently living in this reality, alongside the growing global rates of obesity and diabetes. As a result, MASH is not merely a medical condition but a substantial public health issue. Understanding fibrosis—its progression from MASH and its detection—has therefore become central to contemporary clinical trials.
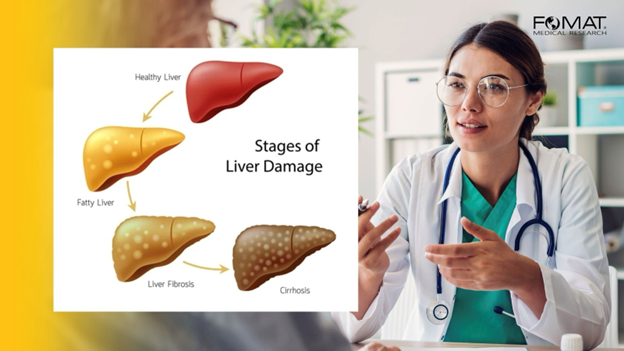
Understanding NASH/MASH and Its Progression to Fibrosis
NASH/MASH is the aggressive form of non-alcoholic fatty liver disease (NAFLD), characterized by liver inflammation and damage caused by fat buildup in the liver. This condition can progress to fibrosis, which is the formation of excessive connective tissue in the liver as a result of the chronic injury. Without intervention, fibrosis may advance to cirrhosis 3 and liver failure. Also Cirrosis can lead into liver cancer ( Hepatocellular carcinoma)4.
The progression from NASH/MASH to fibrosis and beyond is a critical concern because it signifies a transition from a potentially reversible condition to a permanent and life-threatening state. As such, NASH/MASH clinical trials often focus on therapies that can halt or reverse this progression.
Why Fibrosis Is a Key Player in NASH/MASH Trials
- As an Indicator of Disease Progression: The degree of fibrosis in the liver is a strong predictor of future health outcomes in NNASH/ MASH. It helps stratify patients by risk5, tailoring treatments to those who need them most urgently6.
- As a Measure of Treatment Success: In the world of NASH/ MASH trials, the effectiveness of a treatment is often measured by its ability to halt or reverse fibrosis 7.
- As a Target for Therapy: Current research is bustling with activity, focusing on therapies that directly target the processes contributing to fibrosis. Understanding and interrupting these pathways could be key to stopping NASH/ MASH in its tracks.
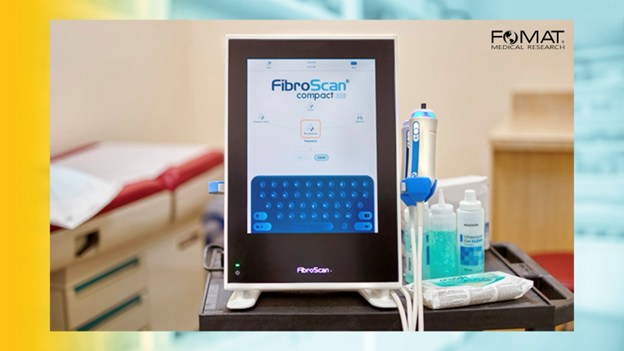
FibroScan in NASH/MASH Clinical Trials
FibroScan, or transient elastography, has become a cornerstone in the evaluation of liver fibrosis in NASH patients8. This non-invasive tool measures liver stiffness, which correlates with the degree of fibrosis.
The advantages of using FibroScan in clinical trials include:
- Non-invasiveness: Unlike liver biopsies, FibroScan is non-invasive and can be performed repeatedly without risk to the patient9. This feature is particularly beneficial in clinical trials where frequent monitoring is necessary.
- Repeatability: The ability to perform repeated assessments without patient discomfort allows for dynamic monitoring of liver stiffness during clinical trials, providing real-time insights into the therapeutic effects of tested drugs.
- Cost-effectiveness and Efficiency: FibroScan is more cost-effective compared to liver biopsy10 and can be conducted quickly11, making it ideal for large-scale screenings in clinical trials.
Challenges and Considerations
While FibroScan offers significant benefits, it also comes with limitations12. Factors such as obesity and the presence of extrahepatic cholestasis can affect the accuracy of liver stiffness measurements13. Furthermore, FibroScan does not differentiate between inflammation and fibrosis, which can sometimes lead to misinterpretations of liver stiffness readings.

Looking Ahead: The Future of Fibrosis Monitoring in NASH/MASH
The medical community is optimistic about refining FibroScan technology and developing new methods to assess liver fibrosis. Imagine biomarkers precise enough to distinguish between mere inflammation and the more sinister fibrosis, or treatments so targeted they can halt fibrosis without side effects.
As we forge ahead, the relentless pace of research and technological innovation heralds a new dawn in combating NASH/MASH. Each clinical trial we conduct and every breakthrough we achieve in understanding fibrosis bring us steps closer to transforming a once daunting diagnosis into a treatable condition.

At FOMAT Medical Research, our ongoing NASH/MASH studies are at the forefront of this fight, seeking to unlock new possibilities for prevention, treatment, and recovery. Participating in our studies not only contributes to crucial scientific progress but also lights the path to a healthier future for countless individuals affected by NASH/MASH.
If you’re ready to take part in this pivotal research and explore the opportunities our studies offer, we invite you to click below to request more information from our dedicated study team. Join us in this silent battle against NASH/MASH and become a beacon of hope in advancing medical science.
References:
- Buzzetti, E., Pinzani, M., & Tsochatzis, E. A. (2021). The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). *Metabolism: Clinical and Experimental, 65*(8), 1038-1048. https://pubmed.ncbi.nlm.nih.gov/34416976/
- National Institute of Diabetes and Digestive and Kidney Diseases. (2021). *Definition & facts for NAFLD & NASH.* https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash/definition-facts#:~:text=Only%20a%20small%20number%20of,of%20U.S.%20adults%20have%20NASH
- National Institute of Diabetes and Digestive and Kidney Diseases. (2021). *Definition & facts for NAFLD & NASH.* https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash/definition-facts#:~:text=NASH%20is%20the%20form%20of,is%20scarred%20and%20permanently%20damaged
- National Cancer Institute. (n.d.). *Types of liver cancer.* https://www.cancer.gov/types/liver
- Paik, J. M., Henry, L., & Younossi, Z. M. (2020). The growing burden of nonalcoholic fatty liver disease in the USA: Its contribution to the rising burden of chronic liver disease. *Hepatology International, 15*(1), 132-139. https://pubmed.ncbi.nlm.nih.gov/33198092/
- Cioni, G., Costa, A., & Ferraiolo, M. (2024). The Role of Ultrasound in the Diagnosis of Non-Alcoholic Fatty Liver Disease. *Diagnostics, 14*(10), 1983. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10696384/
- Loomba, R., & Adams, L. A. (2017). Advances in non-invasive assessment of hepatic fibrosis. *Gut, 66*(11), 1958-1967. https://pubmed.ncbi.nlm.nih.gov/28521870/
- (2023). *Exploring the Complexities of NASH Clinical Trials.* https://www.medpace.com/wp-content/uploads/2023/03/Article-Exploring-the-Complexities-of-NASH-Clinical-Trials.pdf
- Wijarnpreecha, K., Ahmed, A., & Kim, D. (2023). A simple combined score of fibrosis-4 index and NAFLD fibrosis score for assessing advanced fibrosis in non-alcoholic steatohepatitis (NASH). *Alimentary Pharmacology & Therapeutics, 57*(4), 457-463. https://pubmed.ncbi.nlm.nih.gov/36599683/#:~:text=Objective:%20A%20simple%20combined%20score,-alcoholic%20steatohepatitis%20(NASH)
- O’Neil, A. S., Jennings, A. K., Reeve, M. L., Stewart, J. D., Brown, A. G., Smith, C. J., & Cropley, R. (2023). *Sex differences in liver transplantation for NASH.* https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10289790/
- Powell, E. E., Wong, V. W. S., Rinella, M. (2021). Non-alcoholic fatty liver disease. *The Lancet, 397*(10290), 2212-2224. https://pubmed.ncbi.nlm.nih.gov/35090390/
- Younossi, Z., Anstee, Q. M., Marietti, M., Hardy, T., Henry, L., Eslam, M., … & Bugianesi, E. (2020). Global burden of NAFLD and NASH: trends, predictions, risk factors, and prevention. *Nature Reviews Gastroenterology & Hepatology, 16*(1), 20-29. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7047178/
