Using stem cells and gene editing, Salk Institute researchers have unearthed a major driver of aging research, from cancer to diabetes. That driver: loss of heterochromatin, which is potentially reversible as it is an epigenetic process that does not initially forge permanent gene mutations.
“We have identified the loss of heterochromatin as a driver of aging,” geneticist Juan Carlos Izpisua Belmonte, Ph.D., told Bioscience Technology. His team is now “developing new tools for epigenetic editing” with which “we may be able to reverse the epigenetic changes observed during aging and slow down or even reverse the aging process. We are now working on the development of epigenetic editing technologies.”
“The paper convincingly links deletion of the (Werner syndrome RecQ helicase-like) WRN gene to heterochromatin disorganization,” University of California, San Diego, developmental biologist Willis Li, Ph.D., told Bioscience Technology. “It is another example linking heterochromatin loss to aging.” Li’s team, uninvolved in the new work, previously showed in the fruit fly that aging is associated with gradual decline of global heterochromatin levels. He accomplished this by reducing heterochromatin using mutations in a gene called HP1, which accelerated aging, while increasing HP1 boosted heterochromatin levels and extended lifespan. All told, “these results support a heterochromatin loss model for aging,” Li said.
The new study
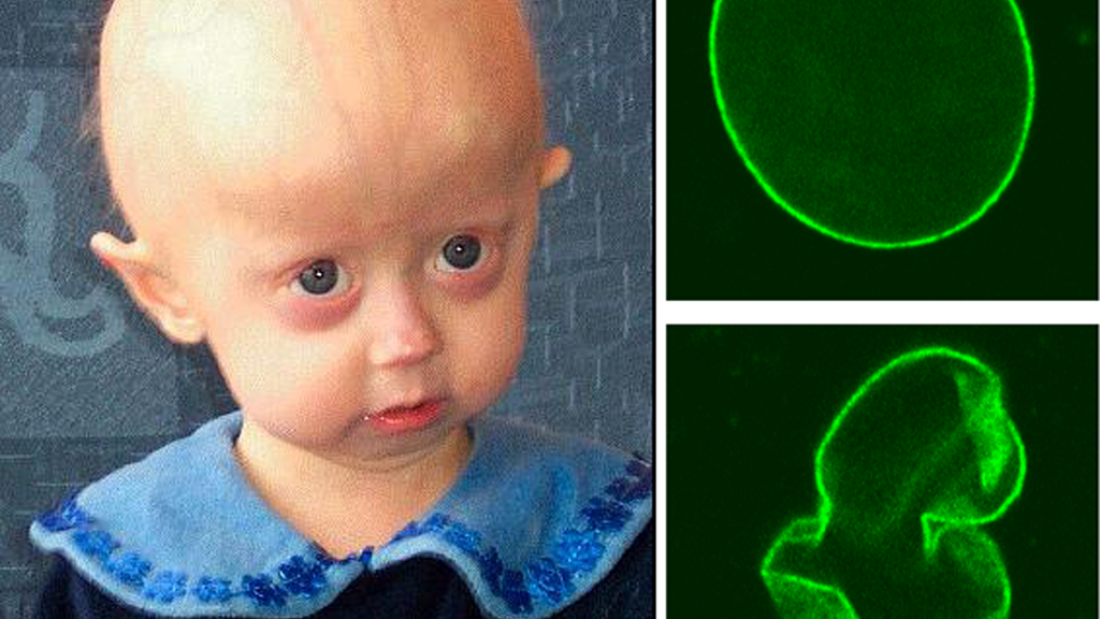
In the April 30 issue of Science, Belmonte’s crew, in association with researchers at the Chinese Academy of Science, discovered that Werner syndrome genetic mutations lead to disruption in heterochromatin, bundles of chromatin that play a major role in controlling gene expression.
Werner syndrome is a devastating illness of premature aging research leading to death in the 40’s and early 50’s. The syndrome is prompted by a mutation in the WRN gene. The healthy gene produces an enzyme responsible for upholding the structure DNA. But when mutated, in Werner syndrome, it interferes with DNA’s normal expansion and repair processes. It has not been known just how the abnormal WRN protein does this.
Belmonte’s crew created a cellular model of Werner syndrome by knocking the WRN gene out of embryonic stem cells (ESCs) differentiated into adult mesenchymal stem cells (MSCs). This knock-out led to disruptions in the heterochromatin, which lie near the cell nucleus.
In additional work, the team revealed the WRN protein interrupts molecular structures well-known to stabilize heterochromatin generally. This created, for the first time, a link between heterochromatin disorganization and the WRN gene, and implicated the natural aging process, too, not just the pathological Werner’s aging.
Most surprising to Belmonte, he told Bioscience Technology, was the notion that WRN is involved in general heterochromatin maintenance. “Previous work has ascribed several functions associated with DNA replication, repair and transcription for the Werner protein—WRN–the DNA helicase mutated in Werner syndrome,” he said. “In addition, WRN is known to play a role in telomere maintenance. For us it was surprising to discover that WRN was interacting with proteins implicated in heterochromatin maintenance.” But it does, they observed. Mutations in WRN led to disruptions in heterechromatin—that drive “the aging process.”
A key for the team seemed to be the choice of ESCs for the model. Other groups have tried to create stem cells, from aged adult Werner cells, called induced pluripotent stem cells (iPSCs). Belmonte’s team investigated some adult Werner iPSCs, but soon discarded them. They were too debilitated to handle all the dedifferentiation and differentiation. “Our model of Werner syndrome is based on the use of ESCs. To generate this model, we eliminated a portion of the WRN protein, mutated in patients with Werner syndrome. The main advantage of this model is the possibility to generate multiple cell types where we can study the aging process.”
He continued: “IPSCs are generated by reprogramming of somatic cells, usually skin cells from a patient. We found that the majority of Werner syndrome patient skin samples available for our studies presented severe karyotype abnormalities and secondary DNA mutations. For these reasons we considered that eliminating a portion of the WRN protein in ESCs will constitute a better model for the study of aging research Werner Syndrome.”
Date: May 5, 2015
Source: http://www.biosciencetechnology.com/articles/2015/05/potentially-reversible-driver-aging-found


Add a Comment